

Viral RNA Isolation: Implement robust RNA extraction methods, such as silica-based columns or magnetic bead technologies, specifically optimized for seminal plasma.
Read More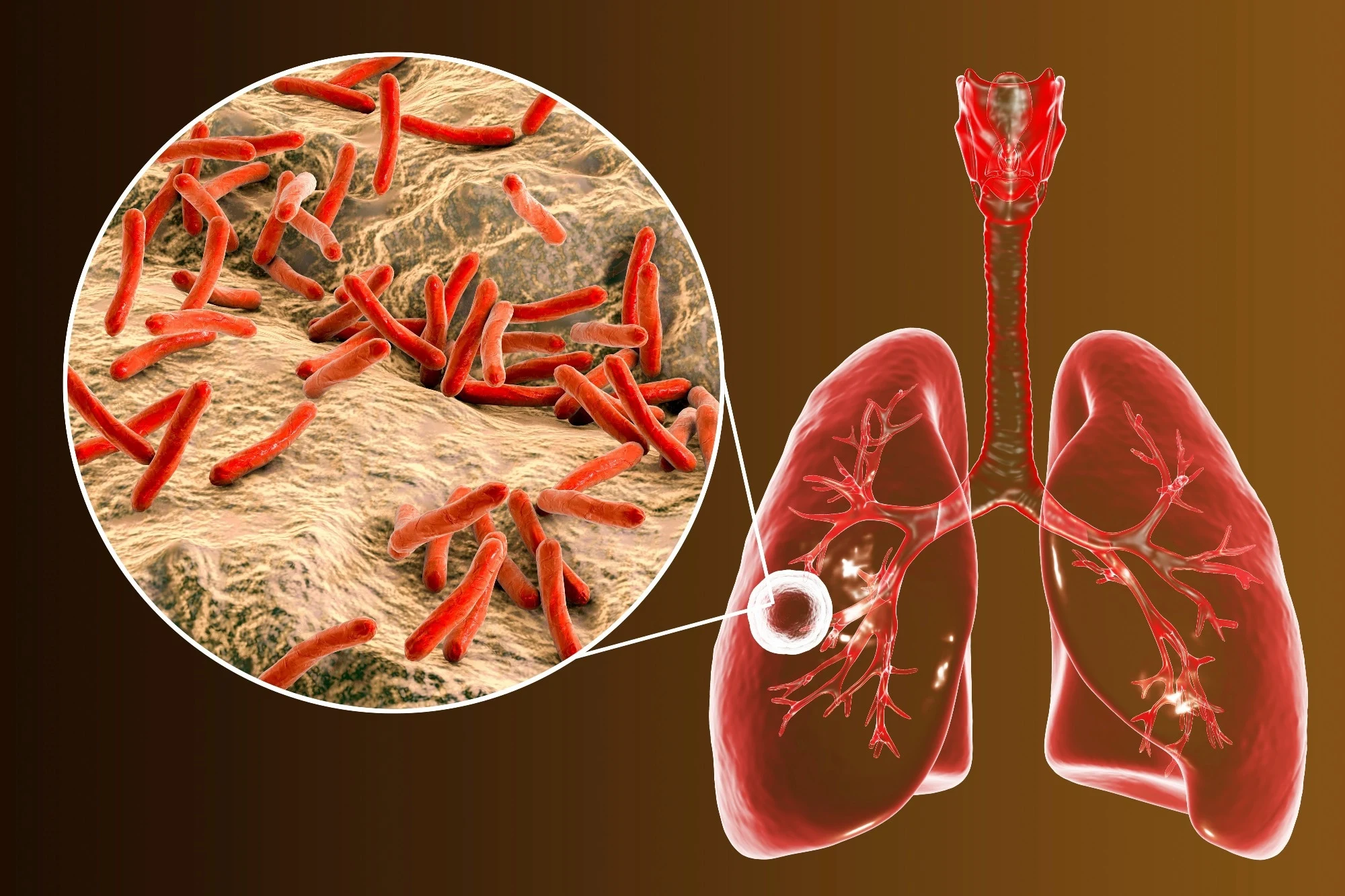
Antimicrobial resistance (AMR) is a growing global health crisis, with multidrug-resistant tuberculosis (MDR-TB) emerging as a significant challenge
Read More
At Suvihaan BioNobel Solutions Pvt. Ltd. (SBS), we are harnessing technology-driven innovation to address critical healthcare challenges.
Read More
• Viral RNA Isolation: Implement robust RNA extraction methods, such as silica-based columns or magnetic bead technologies, specifically optimized for seminal plasma.
• Inhibitor Removal: Samples contains numerous proteins, lipids, and enzymes that may inhibit downstream PCR amplification, so it’s critical to develop optimized inhibitor removal steps (e.g., proteinase K treatment, salting-out techniques, or use of dedicated RNA extraction kits for complex biological fluids).
• Primer Design: Develop highly specific primers and probes targeting viral RNA regions. Special attention is required to design primers that specifically amplify HIV RNA from semen, accounting for variability in seminal plasma composition and the presence of low viral loads.
• Multiplex PCR: Integrate a multiplex PCR approach to simultaneously detect viral RNA levels and drug resistance mutations to offer a comprehensive profile of the virus in the semen sample.
• Quantification: The developed kit must be capable of quantifying HIV RNA levels in seminal fluid. This involves the design of a real-time PCR assay with a quantification standard using known viral RNA concentrations.
• Sensitivity and Specificity: Ensure the kit's sensitivity is optimized for detecting low viral loads, with a limit of detection (LOD) that is sensitive enough for accurate HIV detection in low-volume or poor-quality semen samples.
• Control and Calibration: Integrate internal controls (such as housekeeping genes) and positive/negative controls for accurate results and validation.
• Thermal Cycler Conditions: Optimize the reaction mix, including reagents like primers, probes, buffers, and enzyme concentrations, to suit the complexity of seminal fluid.
• Temperature and Time Optimization: Fine-tune the annealing temperatures and reaction times to achieve maximum yield of the viral RNA.
• Specificity & Cross-Reactivity: Conduct rigorous validation to ensure that the test kit does not cross-react with non-HIV viruses or other pathogens commonly found in semen.
• Sensitivity: Test the kit on semen samples with known viral loads to evaluate the sensitivity and ensure it can detect HIV at low viral levels (a critical requirement for assessing viral shedding in semen).
• Reproducibility: Conduct intra- and inter-laboratory testing to confirm the consistency of results.
• Sample Size: Perform clinical validation studies using a large cohort of HIV-positive individuals and serodiscordant couples to assess the kit's performance in real-world clinical settings.
• Comparison to Plasma HIV Viral Load Testing: Compare the results obtained from the seminal viral load test with plasma-based HIV testing (using existing RT-PCR assays for HIV RNA) to validate the accuracy and clinical applicability.
• Ethical Approval: Obtain ethical clearance from relevant boards (e.g., institutional review boards (IRB)) for trials involving human subjects, ensuring compliance with ethical guidelines for clinical research.
• ISO Certification: Ensure the kit is developed in compliance with ISO 13485 standards for medical device manufacturing and in vitro diagnostics (IVD).
• CE Marking/FDA Approval: Apply for CE marking (Europe) or FDA approval (USA) based on clinical trial results, ensuring the kit meets stringent international standards for diagnostic accuracy and safety.
• Good Manufacturing Practices (GMP): Establish a GMP-compliant manufacturing process for large-scale production of the kits.
• Healthcare Provider Training: Provide training modules for healthcare professionals to ensure correct usage of the test kit and accurate interpretation of results.
• Data Reporting and Integration: Develop a data management system that can integrate results from the viral load test with clinical reports, facilitating patient management and decision-making.
• Market Access: Work with distributors and clinical partners to ensure the test kit is available in hospitals, fertility clinics, and research centers, especially those working with HIV-positive couples and in resource-limited settings.
• Affordability: Ensure the price point is affordable for widespread adoption, particularly in developing countries.
• Monitoring Effectiveness: After market launch, continue monitoring the effectiveness of the kit in clinical settings, including tracking user feedback and real-world performance.
• Ongoing Research: Invest in post-launch research to continually improve the test kit, incorporating new HIV variants and emerging drug resistance patterns into future iterations of the kit.
The development of Viral Load Detection Kits requires a multidisciplinary approach involving molecular biology, advanced diagnostics, and clinical validation. These kits can provide invaluable insights into HIV transmission risks, particularly in reproductive settings, and contribute significantly to better disease management and personalized treatment strategies.
Antimicrobial resistance (AMR) is a growing global health crisis, with multidrug-resistant tuberculosis (MDR-TB) emerging as a significant challenge. MDR-TB, caused by Mycobacterium tuberculosis strains resistant to isoniazid (INH) and rifampicin (RIF), complicates treatment, increases mortality, and poses a substantial burden on healthcare systems.
At Suvihaan BioNobel Solutions Pvt. Ltd., we are pioneering highly sensitive and rapid molecular diagnostics to combat MDR-TB drug resistance through our Multiplex PCR technology. Our innovative multiplex RT-PCR assay is designed to detect multiple drug resistance mutations in a single reaction, enabling faster, more accurate, and cost-effective TB diagnostics.
🔴 Delayed Diagnosis: Traditional culture-based drug susceptibility testing (DST) takes weeks, delaying treatment initiation.
🔴 Emerging Drug Resistance: Extensively drug-resistant TB (XDR-TB) strains are increasingly resistant to second-line drugs.
🔴 High Mortality & Transmission: Delayed treatment results in poor clinical outcomes and increased transmission.
🔴 Limited Diagnostic Accessibility: Many resource-limited regions lack rapid molecular diagnostic solutions.
At Suvihaan BioNobel Solutions Pvt. Ltd., we are transforming MDR-TB diagnostics with our Multiplex PCR-based detection kit, designed for rapid, accurate, and comprehensive identification of drug-resistant tuberculosis strains. Our innovative assay provides early intervention and personalized treatment strategies, ensuring better patient outcomes and global TB control.
• Targets key MDR-TB mutations in rpoB (Rifampicin resistance), katG, and inhA (Isoniazid resistance).
• Identifies second-line drug resistance markers for fluoroquinolones (FQ) and aminoglycosides (AG), assessing the risk of extensively drug-resistant TB (XDR-TB).
• Provides results within 2–3 hours, compared to weeks required for traditional culture-based drug susceptibility testing (DST).
• Works effectively with sputum, bronchoalveolar lavage (BAL), and other clinical specimens for broad clinical application.
• Designed for resource-limited settings, making MDR-TB testing more accessible in high-burden regions.
• Easily integrates into clinical laboratories, reference centers, and national TB control programs for large-scale implementation.
🌍 Early MDR-TB Detection = Improved Patient Outcomes
🔬 Surveillance & Epidemiological Tracking
🏥 Enhancing National TB Programs (NTPs)
Suvihaan BioNobel Solutions is revolutionizing TB diagnostics by integrating AI-driven genomic analysis, enabling:
✔ Automated Resistance Profiling – Personalizing TB treatment regimens through machine learning models.
✔ Real-Time Data Sharing – Enhancing outbreak control efforts by linking with public health agencies.
✔ Portable, Point-of-Care (PoC) MDR-TB Diagnostics – Developing rapid, AI-powered mobile testing solutions for remote and resource-limited settings.
With our Multiplex PCR-based MDR-TB detection kit, Suvihaan BioNobel Solutions Pvt. Ltd. is leading the fight against tuberculosis and antimicrobial resistance. Our commitment to rapid, precise, and scalable molecular diagnostics is helping to:
✅ Reduce TB transmission
✅ Improve treatment outcomes
✅ Strengthen global TB surveillance
Through innovation, research, and cutting-edge technology, we are ensuring a stronger global response against MDR-TB, saving lives and advancing precision diagnostics in infectious disease management.
At Suvihaan BioNobel Solutions Pvt. Ltd. (SBS), we are harnessing technology-driven innovation to address critical healthcare challenges. Our expertise extends beyond traditional molecular diagnostics, integrating artificial intelligence (AI) and mobile health (mHealth) solutions to revolutionize cervical cancer detection and staging.
🔹 Cloud-Based AI Models – For seamless data access and telemedicine applications.
🔹 HPV Risk Assessment Integration – Expanding the app to include real-time HPV risk assessment alongside Pap smear analysis.
🔹 Collaborations with Healthcare Institutions – Partnering with hospitals, government health programs, and NGOs for large-scale implementation.
By combining AI-powered diagnostics with mobile technology, Suvihaan BioNobel Solutions Pvt. Ltd. is transforming cervical cancer screening, staging, and management. Our Android app-based AI system will play a pivotal role in reducing diagnostic delays, improving treatment outcomes, and ultimately saving lives.